A baby milk brand has launched an immediaterecallto add more items following the detection of a harmful bacteria and several children being admitted to hospitals nationwide.
ByHeart, based in Reading, Pennsylvania, is broadening its recall of infant formula to cover all its products after being informed by theFDAconcerning a more extensive inquiry into a recent case of infant botulism.
The FDA notified ByHeart on November 7 about 83 instances of infant botulism reported across the country since August 2025. Currently, the number of cases has risen to 84.
So far, 15 babies have been admitted to the hospital following reports of consuming ByHeart formula.
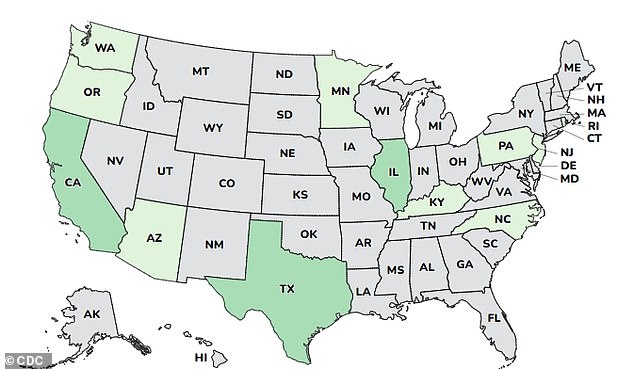
The incidents were documented across 12 states:Arizona, California, Illinois, Minnesota, New Jersey, Oregon, Pennsylvania, Rhode Island, Kentucky, North Carolina, Texas, and Washington.
The FDA stated that no direct link has been established between any infant formula brand and the illness, but health officials are 'concerned that additional batches of ByHeart Whole Nutrition infant formula could be contaminated.'
The California Department of Public Health analyzed a can of ByHeart powdered infant formula that was given to a baby diagnosed with infant botulism, and initial findings indicate the existence of bacteria capable of producing botulinum toxin.
The voluntary recall applies to all batch numbers and all can sizes as well as individual serving packets of ByHeart infant formula.


The CDC recommends that anyone who has the recalled product should note the batch number and 'best before' date if they can.
Parents who have any remaining powdered formula that their baby has consumed should keep it stored properly for a minimum of one month, instead of throwing it away right away.
As per the agency, this measure provides health officials with the necessary time to gather samples if a baby exhibits signs of infant botulism, including difficulty feeding, constipation, or reduced muscle strength.
If no symptoms appear within a month, the remaining formula should be thrown away.
Caregivers are also encouraged to clean and sanitize any objects or surfaces that have been in contact with the recalled formula by using hot, soapy water or a dishwasher, in order to minimize the chance of contamination.
The CDC highlighted that these actions are part of a larger initiative to assist the FDA's continuous inquiry and ensure the well-being of babies as the origin of the outbreak is being investigated.
Infant botulism is an uncommon yet severe illness that impacts infants, typically those younger than 12 months.
In the United States, approximately 200 to 300 instances of infant botulism are reported annually. Most of these, about two-thirds, involve infant botulism, which primarily impacts children younger than one year of age.
This occurs when spores of the bacterium Clostridium botulinum get into an infant's digestive system, where they may multiply and release botulinum toxin — one of the strongest naturally occurring poisons.
Symptoms may involve constipation, inadequate feeding, drooping eyelids, a weak cry, reduced muscle tone, and in serious instances, breathing problems or cessation.
In cases of infant botulism, the food does not carry the toxin itself; rather, it contains spores capable of generating toxin within the baby's system.

The most recognized food associated with infant botulism is honey, and medical experts recommend avoiding giving it to children younger than 12 months.
Sometimes, spores may also be present in dusty indoor settings, uncleaned fruits, or powdered food items, although these are less common ways to come into contact with them.
The primary treatment for infant botulism involves a medication known as Botulism Immune Globulin Intravenous (Human), referred to as BIG-IV, given through one intravenous injection.
Supportive care is also crucial, which can involve hospitalization, the use of a ventilator for breathing assistance if required, and intravenous fluids or tube feeding to ensure proper nutrition when the baby struggles to swallow.
Timely detection and intervention play a vital role in achieving a positive result.
Although death is uncommon, occurring in less than one percent of cases, recovery may take a long time, often requiring several months or even years to fully recover.
As of now, there have been no recorded fatalities due to the outbreak.
The organization has noted that the recall is taking place due to an excess of caution, as the FDA has not confirmed a direct connection between infant formula and the reported instances of botulism.

There is also no historical example of infant formula leading to infant botulism.
"None of the ByHeart products have shown positive results for any contaminants," stated Mia Funt, Co-Founder and President of ByHeart.
The topmost concern of ours is the safety and health of each baby who consumes our formula.
Although there has been no verification of contamination, this voluntary recall demonstrates our strong dedication to openness and the well-being of infants and their caregivers.
Funt mentioned that ByHeart adheres to the top international and U.S. safety standards and testing procedures for its products.
Verification for certain cases is still in progress.
Individuals who bought items from the affected batches have been told to stop using and throw away the product immediately. ByHeart will exchange any recalled cans without charge.
Parents are advised to reach out to their doctor if their baby exhibits signs that may indicate botulism.
In the meantime, companies have been directed to cease selling the recalled ByHeart Whole Nutrition infant formula and to clean any surfaces that might have come into contact with the product.
Read more- Why did ByHeart choose to recall their baby formula voluntarily during the FDA's larger botulism inquiry, despite no confirmed connection?
- Is your child's well-being in danger due to widely used baby food being removed across the country because it contains excessive levels of lead?
- What concerning results prompted the withdrawal of more than 50,000 baby food packets from Publix displays?
- Underlying health risk: Might bacteria found in your butter result in deadly germs? Discover more about this concerning product recall!
- What well-known nutritional shakes are involved in a dangerous listeria contamination that has led to a country-wide product recall?
